After this long experimental process it is time to reflect.....
What would I change in my experiment if I could complete it again?
- I would conduct more background research to broaden my knowledge, relating to my experimental hypothesis, before commencing the practical phases
- I would have been more organised in gathering all materials and items needed so my experiment could be more accurate
- I would repeat my experiment a further 2 times to ensure the experimental results are
reliable
- I would have taken more time when measuring different materials to improve the validity of my experiment and may have considered filtering the liquids at home before the experimental lesson to save time
- I would have been more organised in gathering all materials and items needed so my experiment could be more accurate
- I would repeat my experiment a further 2 times to ensure the experimental results are
reliable
- I would have taken more time when measuring different materials to improve the validity of my experiment and may have considered filtering the liquids at home before the experimental lesson to save time
The outcomes for this experiment where overall successful.
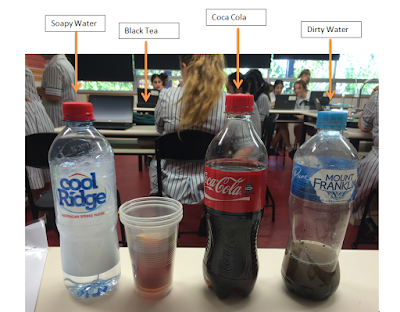
I measured turbidity of the filtered and non-filtered liquids and then calculated and graphed the difference between the average turbidity’s of the filtered and non-filtered liquids. At the end of my experiment the average difference between the filtered and non-filtered dirty water was 671.2NTU, the black tea was 84NTU, the Coca-Cola was 56.5NTU and the soapy water was 131.1NTU. The turbidity of the dirty water will change the most drastically when it is filtered, followed by the soapy water, then the black tea and finally the cola-cola. There is not evident trend from my results although, it can be assumed that the turbidity’s of the liquids that had larger particles in them (such as the dirty water and the soapy water- dirty particles and bubbles made from the detergent) changed more drastically when filtered than the liquids (Coca-Cola and black tea) in which had dissolved particles in them.
Conclusion:
In conclusion,
the effective water filter successfully filtered different liquids, causing
the turbidity of the liquid to change. The turbidity of the dirty water changed
the most drastically when it was filtered followed by the soapy water, then
the black tea and finally the cola-cola, therefor proving my hypothesis to be
correct.
Overall I would say this was a successful project and I am very pleased with the result. Thank you for being a part of my Science Experiment for 2016.